
Foreign Certificate of Approval signed by the Massachusetts Secretary of State, if the organization is not registered in Massachusetts.Articles of Incorporation or Partnership approved by the Massachusetts Secretary of State or.

*The Clinical Laboratory Disclosure of Ownership Interest Statement was updated February 1, 2019.
#CLINICAL LABORATORY LICENSE VERIFICATION CALIFORNIA FULL#
The following documents must be completed in full as part of the clinical laboratory licensure application packet.
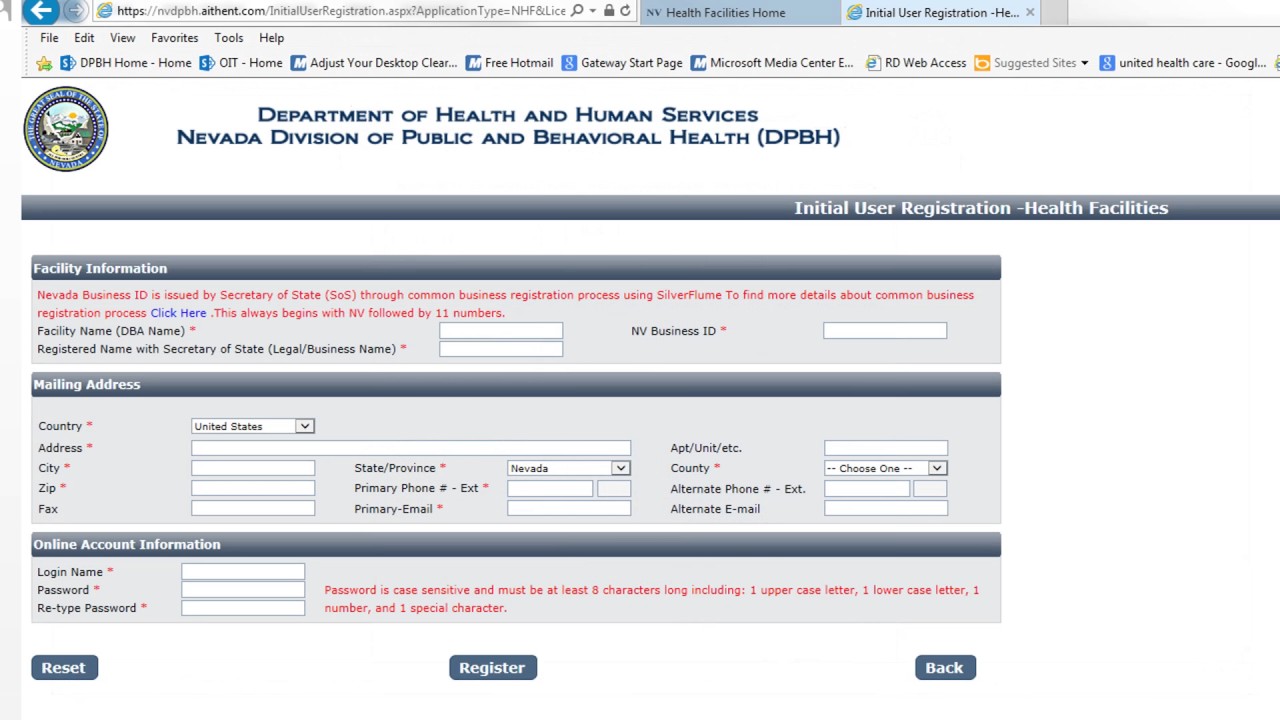

The laboratory must be enrolled under the same name that will appear on the license and must authorize the proficiency testing service to release all test results to the Clinical Laboratory Program.Ī laboratory that is already licensed and wishes to add a specialty or subspecialty must notify and obtain approval from DPH before starting testing.įor more information about clinical laboratory licensure in Massachusetts, please see the frequently asked questions about clinical laboratory state licensure page. A clinical laboratory that performs testing and requires licensure must obtain a license from the Department of Public Health (DPH) before starting testing.īefore applying for Massachusetts state licensure, a clinical laboratory must participate in an approved proficiency-testing program that covers all laboratory analytes for which proficiency testing is available.


 0 kommentar(er)
0 kommentar(er)
